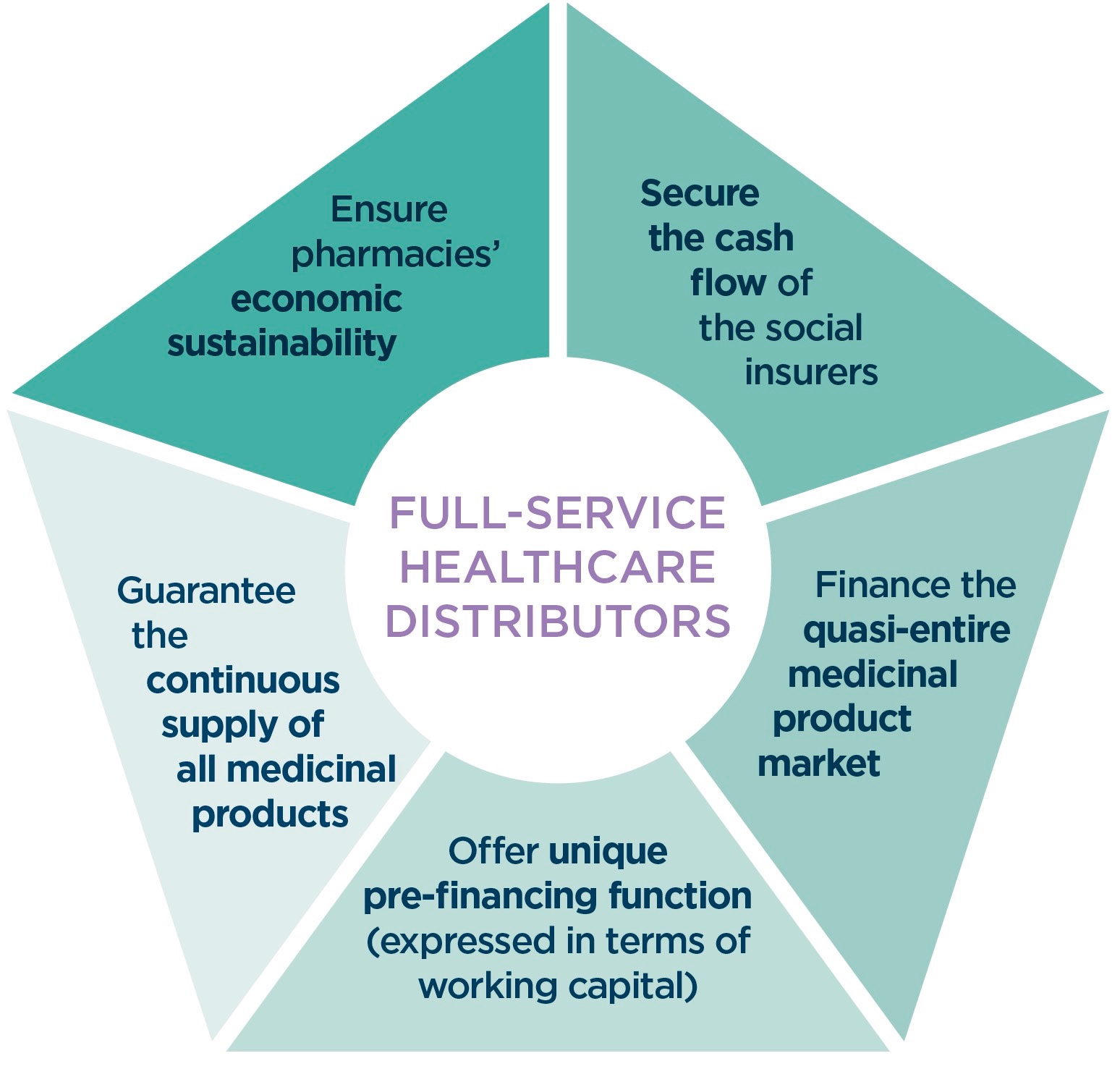
The role and value of full-service healthcare distributors
GIRP and its members advocate for sustainable solutions which prioritise medicines availability, accessibility and affordability by outlining the core function of full-service healthcare distributors (also referred to as pharmaceutical full-line wholesalers), being the vital link for the fair, efficient, timely and safe distribution of all medicinal products, including medical devices and other medical supplies, to patients across Europe.
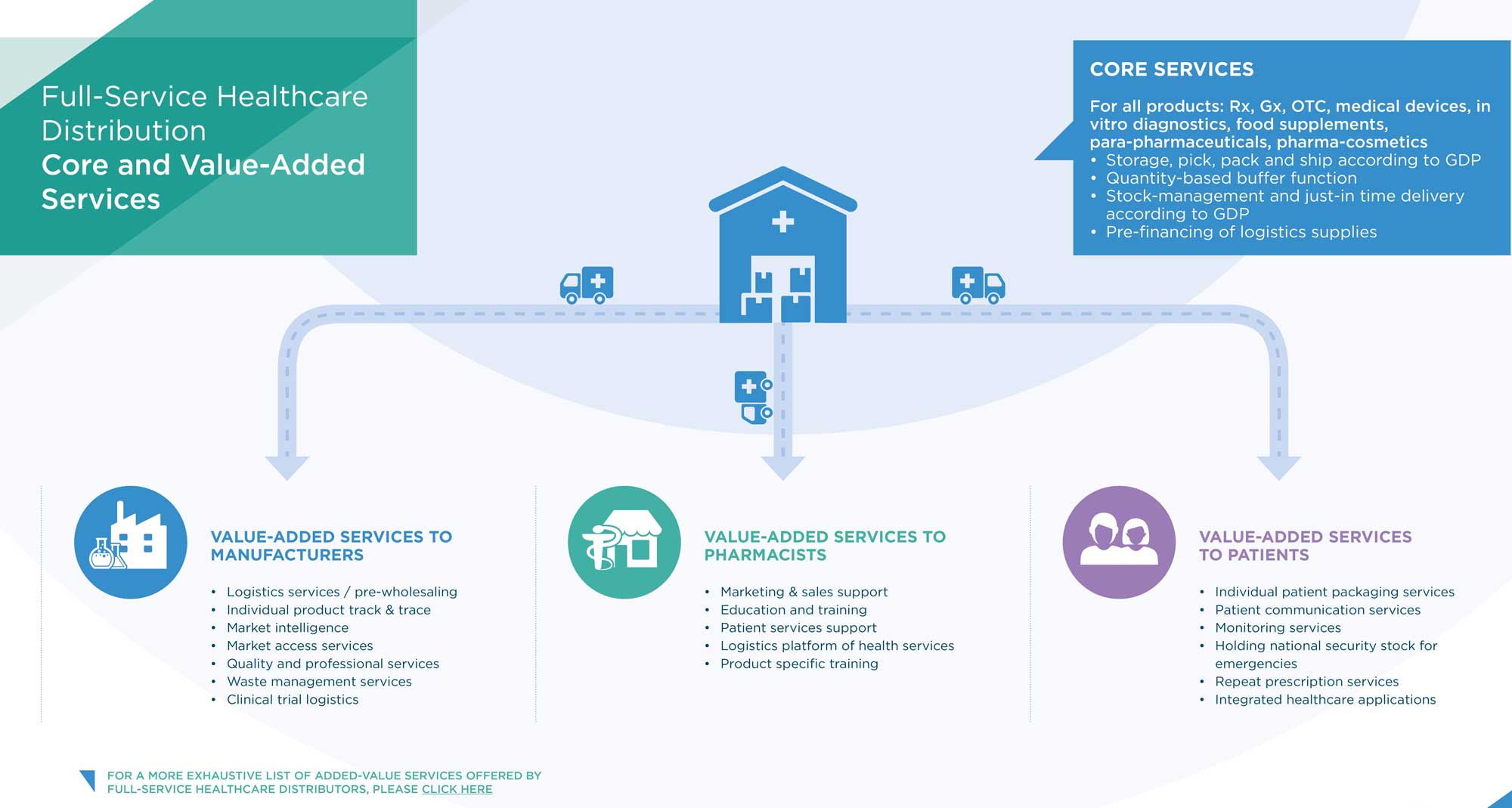
Full-service healthcare distributors carry the complete assortment of medicines required by pharmacies/patients in their country. They have the infrastructure to store and keep medicines safely and under clinically required conditions until needed by hospitals, pharmacies and doctors in all locations across countries.
Full-service healthcare distributors adhere to and promote the highest standards of Good Distribution Practice (GDP) and where applicable Good Manufacturing Practices (GMP). They ensure the highest level of compliance to all applicable regulatory standards, including the Falsified Medicines Directive and its Delegated Regulation on Safety Features. Furthermore, they provide a wide range of added value services to manufacturers and pharmacies to the benefit of patients and the public.

Full-service healthcare distributors are indispensable and crucial components of European healthcare systems. While navigating a highly complex regulatory environment, they supply around 200,000 dispensing points with more than 15 billion packs of medicines per year – equating to 62 million packs every single day.
Full-service healthcare distributors typically work out of public sight, yet bring tremendous value to patients and healthcare systems. Patients trust that their local pharmacy and other healthcare institutions will have the correct medicine in the exact quantity and at the precise time needed. Patience rely on pharmaceutical full-line wholesalers to continuously provide a rapid, safe and manufacturer-independent supply of medicines, including lifesaving ones, to the sites of healthcare provision.
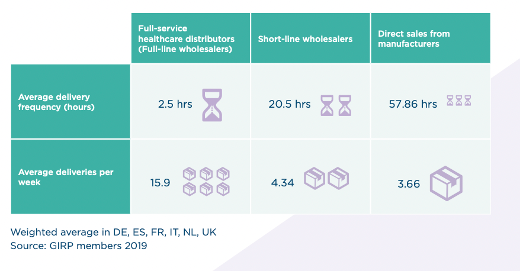
The activity of full-service healthcare distributors consists of the purchase, warehousing, storage, order preparation, sale and delivery of medicines. Pharmaceutical full-line wholesalers carry and distribute the complete assortment of products in range and depth within the framework set by national authorities and the market to meet the needs of those with whom they have business relations and deliver all medicines in their geographical area of activity within a very short time (average delivery time within the 6 largest EU countries is around 2.5 hours).

Full-service healthcare distributors are committed to offer a one-stop shop solution for their customers’ needs. Their core driving value is to supply their customers with sufficient stock.
Not only do they have a sense of duty and responsibility to deliver the medicines needed to their daily customers, but it is also a rational business approach to ensure they are sufficiently supplied. No full-service healthcare distributors would ever wish to be in the position to inform its customers of an inability to supply and therefore will do its uttermost to meet the demand.
Full-service healthcare distributors fulfil a vital public service role and function.
Our governing bodies cooperatively oversee the activities of GIRP for the evaluation of recent developments and for the distinction of new objectives. These bodies guide the association’s direction and work on improving the organisation by setting concrete outlines, goals and benchmarks. The constitution and workflow of GIRP’s decision-making bodies follows democratic principles and GIRP actively promotes dialogue and consensus in its decisions.
- Directors of Associations Committee
- Economic and Social Affairs Committee
- eHealth Committee
- Advisory Council Supply Chain Solutions Committee
- Technical Committee
- Legal Affairs Committee
- Public Affairs and Public Communications Committee
- Green Deal Working Group
- Pharmaceutical Overhaul Working Group
- Sub-group on Shortages Monitoring systems
- Ad-hoc Shortages working Group
- European Healthcare Value Chain Network
- Retail Chain Network
